Wang Sibao Lab
Our Research
Insects constitute more than half of all known living organisms and play diverse roles in ecosystems, agriculture, and human health. Through long-term co-evolution, insects and microbes have developed complex and dynamic interactions—including mutualistic symbiosis, pathogenic parasitism, immune defense, and immune evasion. Phytophagous insects cause crop losses through foliar feeding, while vector insects transmit a wide range of pathogens to humans, animals, and plants, posing serious threats to public health, food security, and ecological balance.
Mosquitoes, in particular, are vectors for numerous human pathogens that cause devastating diseases such as malaria, dengue, yellow fever, and Zika. Malaria alone remains one of the leading causes of morbidity and mortality in tropical and subtropical regions.
Our laboratory focuses on deciphering the molecular mechanisms underlying mosquito–pathogen–environment interactions. We aim to advance a mechanistic understanding of the dynamic interplay between mosquitoes, gut microbiota, pathogens, and environmental cues. Ultimately, our goal is to translate this knowledge into innovative, sustainable strategies for pest management and the prevention of vector-borne diseases.
To achieve this, we employ an integrative and interdisciplinary research framework that combines molecular biology, epigenetics, cell biology, immunology, chemical ecology, microbiome, synthetic biology, evolutionary biology to investigate the following research directions:
1. Mosquito immunity and pathogen transmission
We have been investigating how mosquitoes and other insect vectors recognize, respond to, and control infections by malaria parasites and arboviruses. We have been exploring the host factors that are exploited by Plasmodium and arboviruses for their invasion, development, and replication within the mosquito hosts.
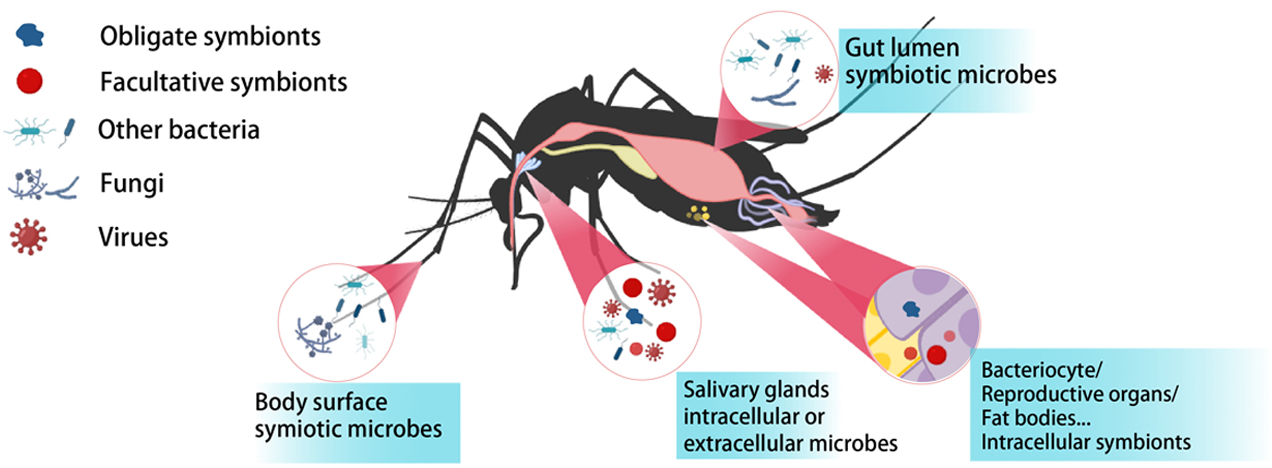
2. Molecular interactions between host insects, gut microbiota, and entomopathogens
We study how symbiotic bacteria colonize the mosquito gut, maintain gut homeostasis, modulate host immunity and metabolism, and influence pathogen infection and vector competence. We have been studying molecular interactions between insects and entomopathogenic fungi or bacteria, focusing particularly on the regulatory roles of small RNAs, histone modifications, and other epigenetic mechanisms in shaping these host–pathogen relationships.
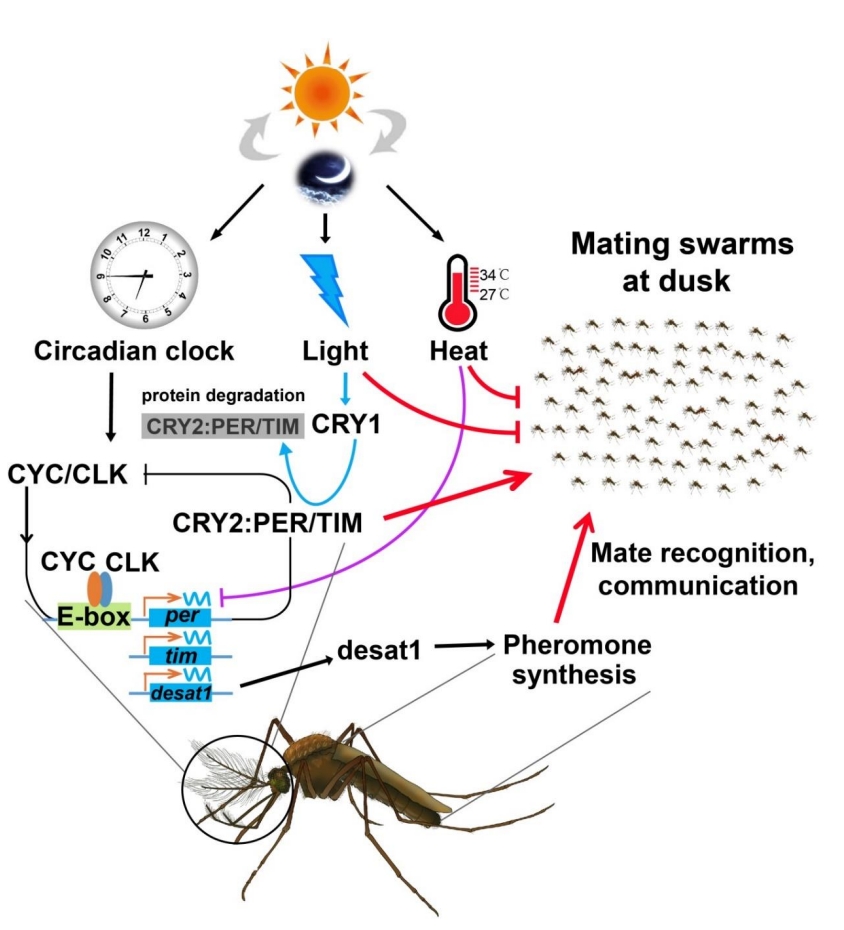
3. Mosquito mating biology and adaptive evolution
We have been deciphering the molecular underpinnings of mosquito mating flight swarming and mating behavior, and investigating how mosquitoes adapt to environmental changes such as rising temperatures.
-

Mosquito adults
-

Mosquito larva
-

Mosquito pupa
-

Mosquito eggs
Research Progress
Mosquito microbiome
We have developed innovative strategies to render mosquitoes incompetent vector by introducing anti-pathogen symbiotic bacteria into mosquito populations (Science, 2017; Nat Commun, 2025; PLoS Pathogen, 2025); revealed how natural commensal bacteria directly inhibit malaria parasites by producing antimalarial effectors and employ quorum sensing-regulated extracellular vesicles to deliver effector proteins to the parasite for targeted killing (Nat Microbiol 2021; Cell Host & Microbe, 2023; Nat Commun, 2023). These findings opened new avenues for the development of symbiont-based vector-borne disease control.
Mosquito swarming and mating
We have revealed how clock genes and environmental cues coordinate Anopheles mosquito swarming, mating, and pheromone production. This research led to the identification of the first-known mosquito pheromone and provided crucial insights into the chemical and behavioral ecology of mosquito reproduction, providing theoretical basis for improving genetic control strategies by enhancing mating competitiveness of released male mosquitoes (Science, 2021).
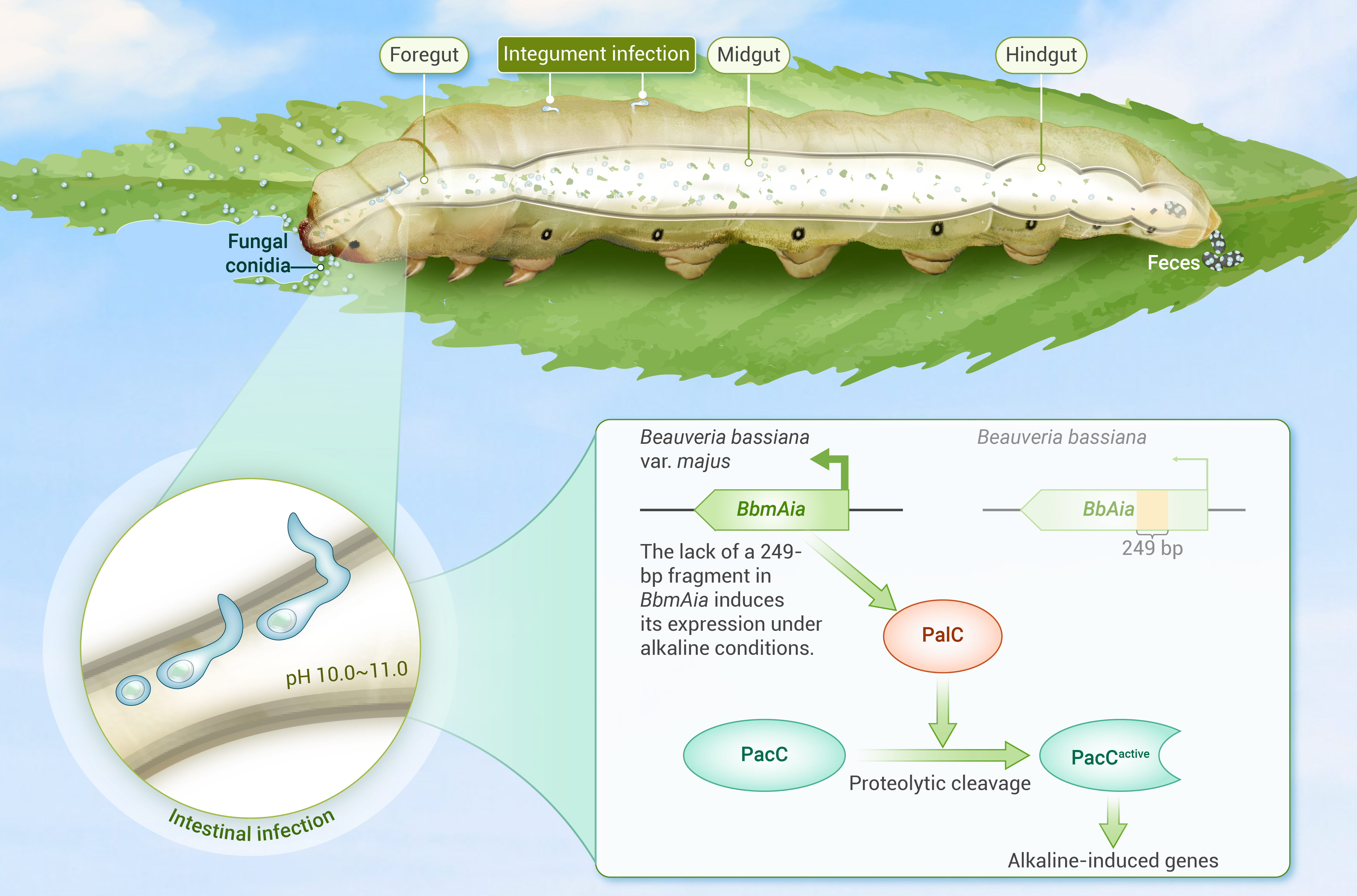
Insect-entomopathogenic fungi interactions
We have identified crucial epigenetic factors that regulate appressorium formation and turgor generation in entomopathogenic fungi, and elucidated their target genes and the associated genetic regulatory networks (Science Advances, 2020; PNAS, 2023), discovered sRNA-mediated cross-kingdom regulation in insect-fungal pathogen interactions (Nat Commun, 2019; PNAS, 2021), and developing a novel approach to enhance the efficacy of fungal insecticides by exploiting host microRNAs (Cell Reports, 2022). We discovered the insect foregut as an alternative infection route for fungal entomopathogens (The Innovation, 2024).