Welcome to Mao lab!
Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance
Abstract Immunity deteriorates with age in animals but comparatively little is known about the temporal regulation of plant resistance to herbivores. The phytohormone jasmonate (JA) is a key regulator of plant insect defense. Here, we show that the JA response decays progressively in Arabidopsis. We show that this decay is regulated by the miR156-targeted SQUAMOSA PROMOTER BINDING PROTEIN-LIKE9 (SPL9) group of proteins, which can interact with JA ZIM-domain (JAZ) proteins, including JAZ3. As SPL9 levels gradually increase, JAZ3 accumulates and the JA response is attenuated. We provide evidence that this pathway contributes to insect resistance in young plants. Interestingly however, despite the decay in JA response, older plants are still comparatively more resistant to both the lepidopteran generalist Helicoverpa armigera and the specialist Plutella xylostella, along with increased accumulation of glucosinolates. We propose a model whereby constitutive accumulation of defense compounds plays a role in compensating for age-related JA-response attenuation during plant maturation.
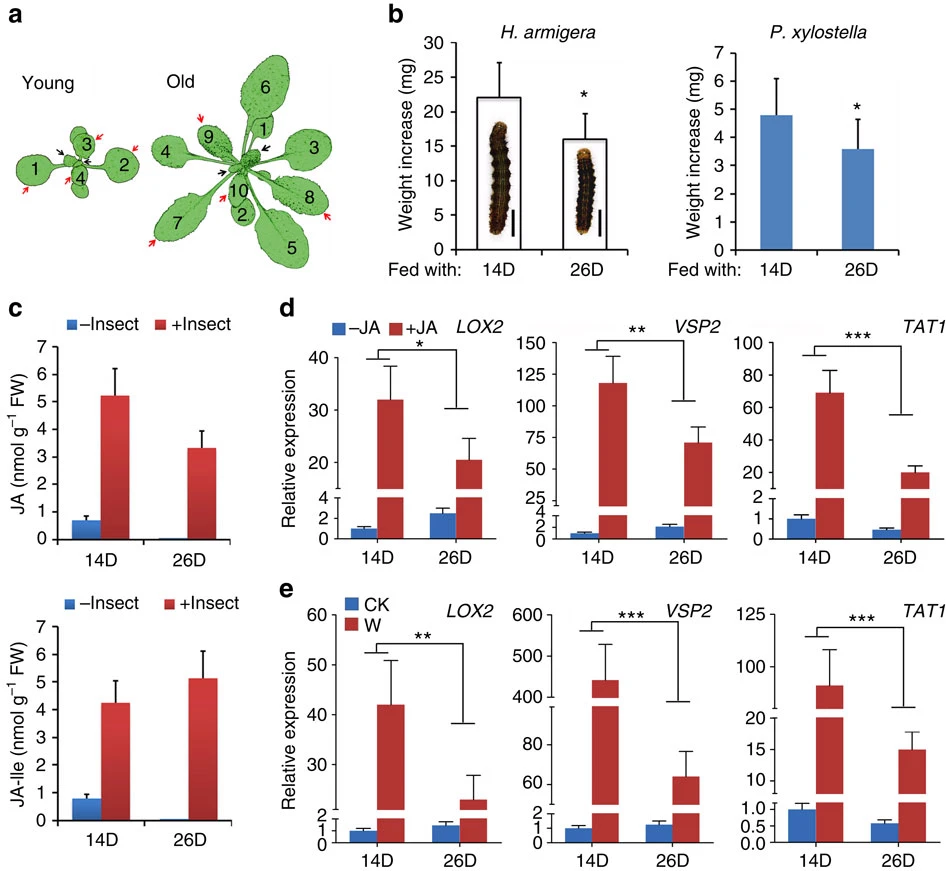
Figure 1. (a) Diagrams of young (14-day-old, 14D) and old/adult (26-day-old, 26D) plants of Arabidopsis thaliana (Col-0) grown in LD. Black arrows indicate the newly initiated leaves (new leaves, ∼3 mm in width), and red arrows indicate the rapidly expanding leaves. (b) Weight increase of H. armigera and P. xylostella larvae fed with rapidly expanding leaves harvested from the indicated plants for 3 days, both gained less weight from the old (26D) plants. Data are means ±s.d. (n=25), asterisk indicates significant difference from the 14D group (Student’s t-test, *P<0.05). Embedded in the column is the image of the H. armigera larva after feeding. Scale bar, 1 cm. (c) Analysis of JA and JA-Ile contents in young (14D) and old (26D) plants by UPLC-MS. Plants were challenged by H. armigera third instar larvae for 12 h and the rapidly expanding leaves were collected for analysis. Intact plants were used as control. Data are means ±s.d.(n=3). (d,e) Expression of LOX2, VSP2 and TAT1 in young (14D) and old (26D) plants in LD. Transcript levels were detected by qRT-PCR. Data were analysed by multiple comparisons (Tukey test) followed by two-way ANOVA (*P<0.05, **P≤0.01, ***P≤0.001). Error bars represent ±s.d. (n=3). (d) Gene expressions in total aerial tissues of young (14D) and old (26D) plants 4 h post-MeJA treatment, the JA response attenuated with plant age. The expression in the 14D control plants (−JA) was set to 1. (e) Gene expressions in rapidly expanding leaves of the young (14D) and the old (26D) plants 2 h post-wounding (W) treatment, the wounding response attenuated with plant age. The expression in the 14D intact plants (CK) was set to 1. ANOVA, analysis of variance; UPLC-MS, ultra performance liquid chromatography-mass spectrometry.
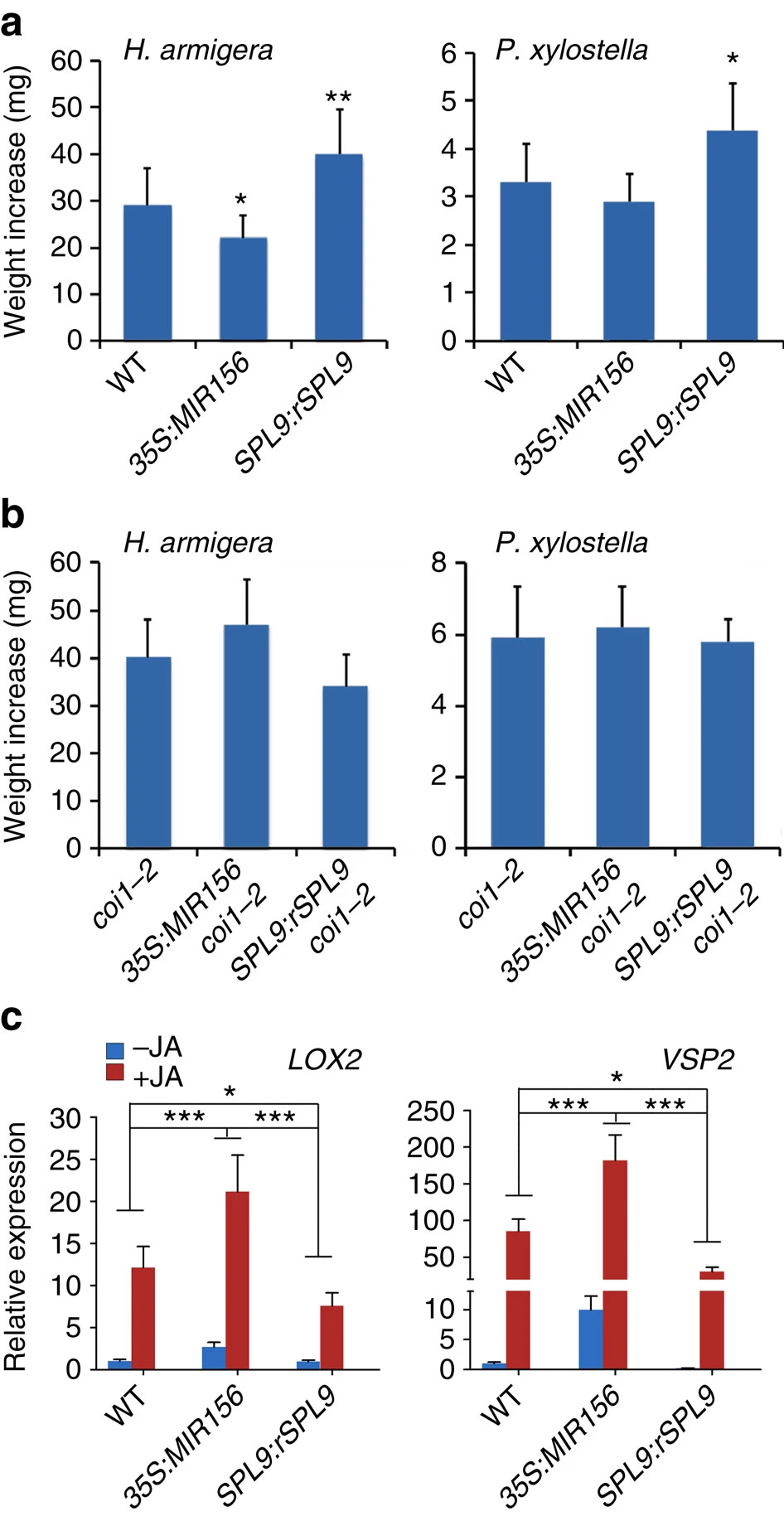
Figure 2. (a,b) Weight increase of H. armigera and P. xylostella larvae fed with the indicated plant leaves, both insects gained higher weight from the SPL9:rSPL9 plants. Leaves from the ∼30-day-old plants in SD were used in feeding. Data are means ±s.d. (n=25), asterisks indicate a significant difference from the wild-type (WT) group (Student’s t-test, *P<0.05, **P<0.01). (c) LOX2 and VSP2 expressions in plants over-expressing miR156 (35S:MIR156) or SPL9 (SPL9:rSPL9). Plants (30-day-old in SD) were treated with 50 μM MeJA (+JA) or ethanol (−JA) as control, and 4 h later the transcript levels in new leaves of the indicated plants were detected by qRT-PCR. The expression in the wild-type was set to 1. Data were analysed by multiple comparisons (Tukey test) followed by two-way ANOVA (*P<0.05, ***P≤0.001). Error bars represent ±s.d. (n=3). ANOVA, analysis of variance.
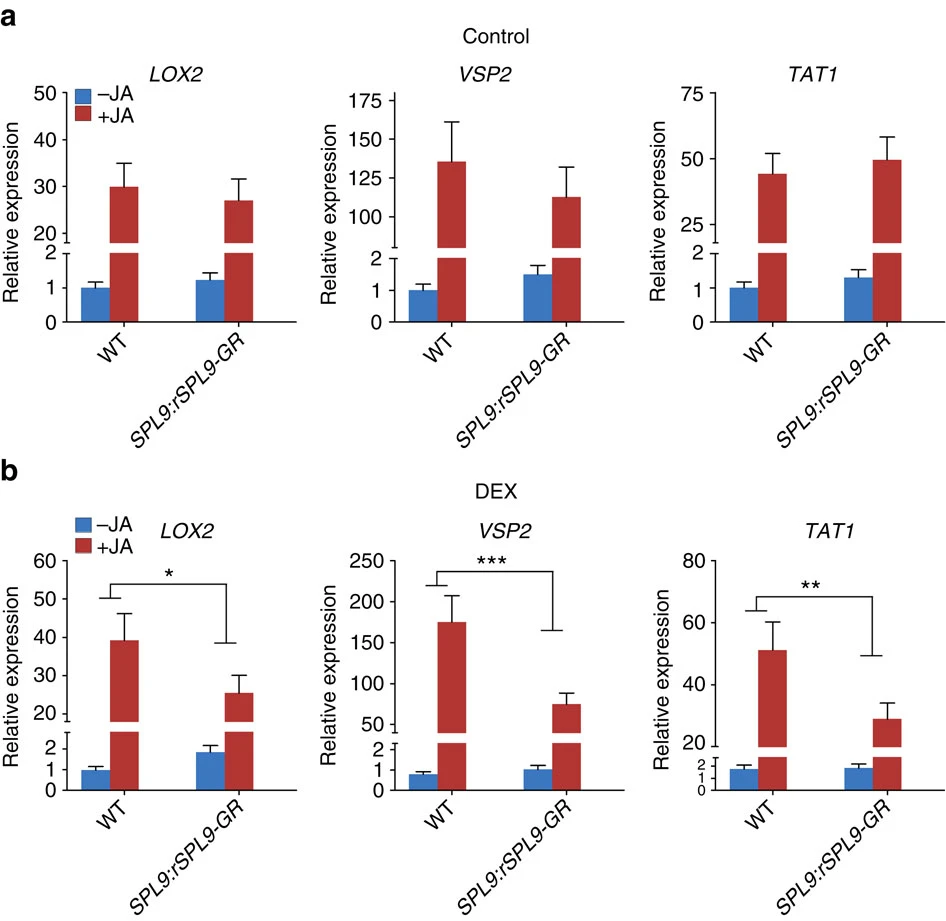
Figure 3. The wild-type and SPL9:rSPL9-GR plants (12D in LD) were first sprayed with ethanol (control) (a) or 10 μM DEX (b), and after 12 h the plants were treated with 50 μM MeJA (+JA) or ethanol (−JA). Four hours later the transcript levels in the first pair of leaves were detected by qRT-PCR. The expression in the wild-type free from DEX and JA was set to 1. Data were analysed by multiple comparisons (Tukey test) followed by two-way ANOVA (*P<0.05, **P≤0.01, ***P≤0.001). Error bars represent ±s.d. (n=3). ANOVA, analysis of variance.
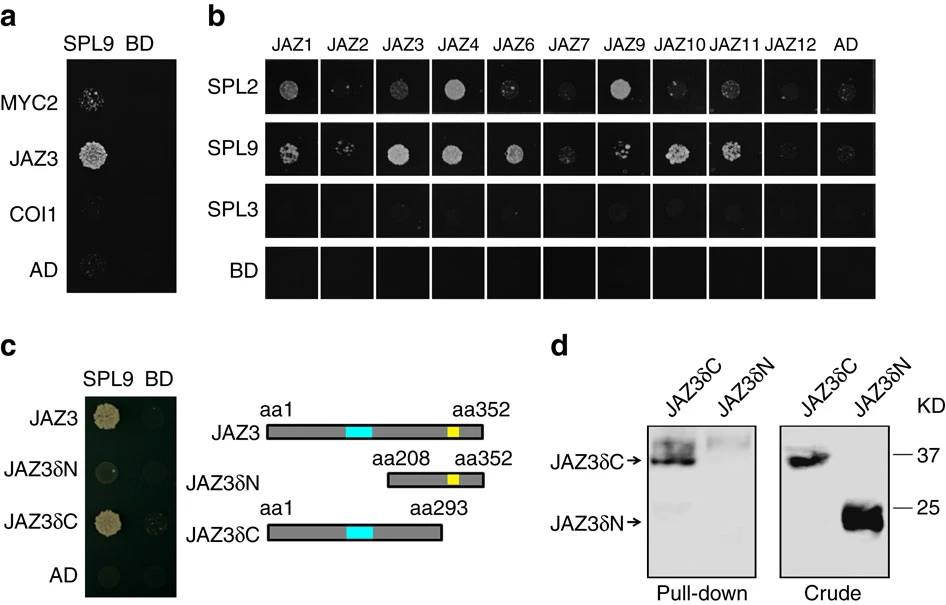
Figure 4.Yeast two-hybrid assay. SPLs were fused to GAL4 DNA-binding domain (BD), MYC2, COI1, JAZ3, JAZ3δN and JAZ3δC was fused to GAL4 activation domain (AD), respectively. Interactions were examined with 10 mM (for SPL2 and SPL3) or 15 mM (for SPL9) 3-amino-1,2,4-triazole. Schematic diagrams of truncated versions of JAZ3 are shown in c, blue box indicates ZIM domain and yellow box indicates Jas domain. SPL9 and SPL2, but not SPL3, interacted with JAZs (a,b), and the N-terminal of JAZ3 was responsible for binding to SPL9 (c). (d) Pull-down assay of JAZ3-SPL9 binding. Recombinant HIS-SPL9 protein was incubated with total proteins of the tobacco leaf expressing either JAZ3δN-HA or JAZ3δC-HA driven by the 35 S promoter. Anti-HA antibody was used to detect the truncated fusion proteins of JAZ3 before (Crude) or after (Pull-down) immunoprecipitation. KD, kilodalton.
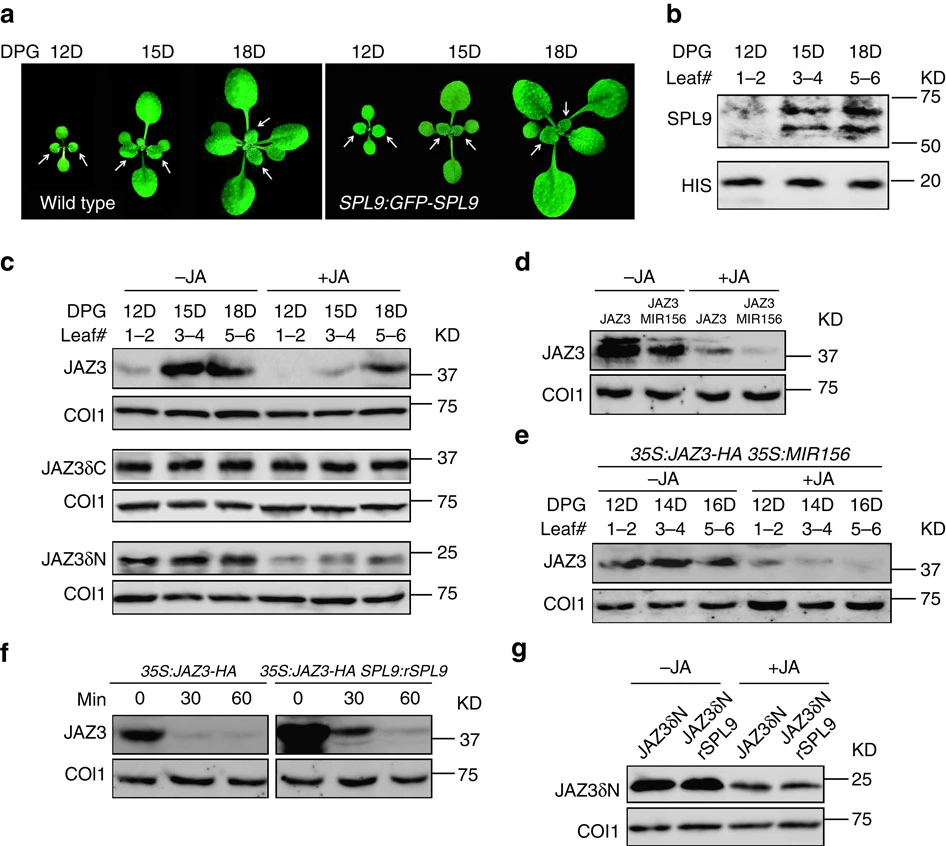
Figure 5. (a) Images of wild-type and SPL9:GFP-SPL9 plants in LD at indicated days post germination (DPG). White arrows indicate new leaves (leaf #1–2, 3–4 and 5–6). (b–g) Protein levels of SPL9 and JAZ3 in new leaves. KD, kilodalton. (b) SPL9-GFP fusion protein in new leaves as described in a were detected by anti-GFP antibody, the protein level increased with plant age. (c–g) JAZ3-HA accumulation with plant age. Plants in LD were treated with 50 μM MeJA (+JA) or ethanol (-JA), and new leaves were collected one hour later or at the indicated time post treatment for immunoblotting. JAZ3-HA or the truncated versions were detected using anti-HA antibody. COI1 in each sample was detected using anti-COI1 antibody. (c) JAZ3-HA (top), JAZ3δC-HA (middle) and JAZ3δN-HA (bottom) proteins in leaves (leaf #1–2, 3–4 and 5–6) harvested from 35S:JAZ3-HA, 35:JAZ3δC-HA and 35S:JAZ3δN-HA plants at the indicated DPG. The JAZ3-HA fusion protein, but not its truncated versions, exhibited the age-dependent accumulation. (d) JAZ3-HA level was decreased in the 35S:MIR156 background. The first pair of leaves from 35S:JAZ3-HA (JAZ3) and 35S:JAZ3-HA 35S:MIR156 (JAZ3 MIR156) plants were collected for analysis. (e) JAZ3-HA level was similar among leaves (leaf #1–2, 3–4 and 5–6) harvested from 35S:JAZ3-HA 35S:MIR156 plants at the indicated DPG. (f) JAZ3-HA level was increased in the SPL9:rSPL9 background. The first pair of leaves from 35S:JAZ3-HA and 35S:JAZ3-HA SPL9:rSPL9 plants were analysed. (g) JAZ3δN-HA protein level in 35S:JAZ3δN-HA and 35S:JAZ3δN-HA SPL9:rSPL9 plants, which was uncoupled from SPL9.
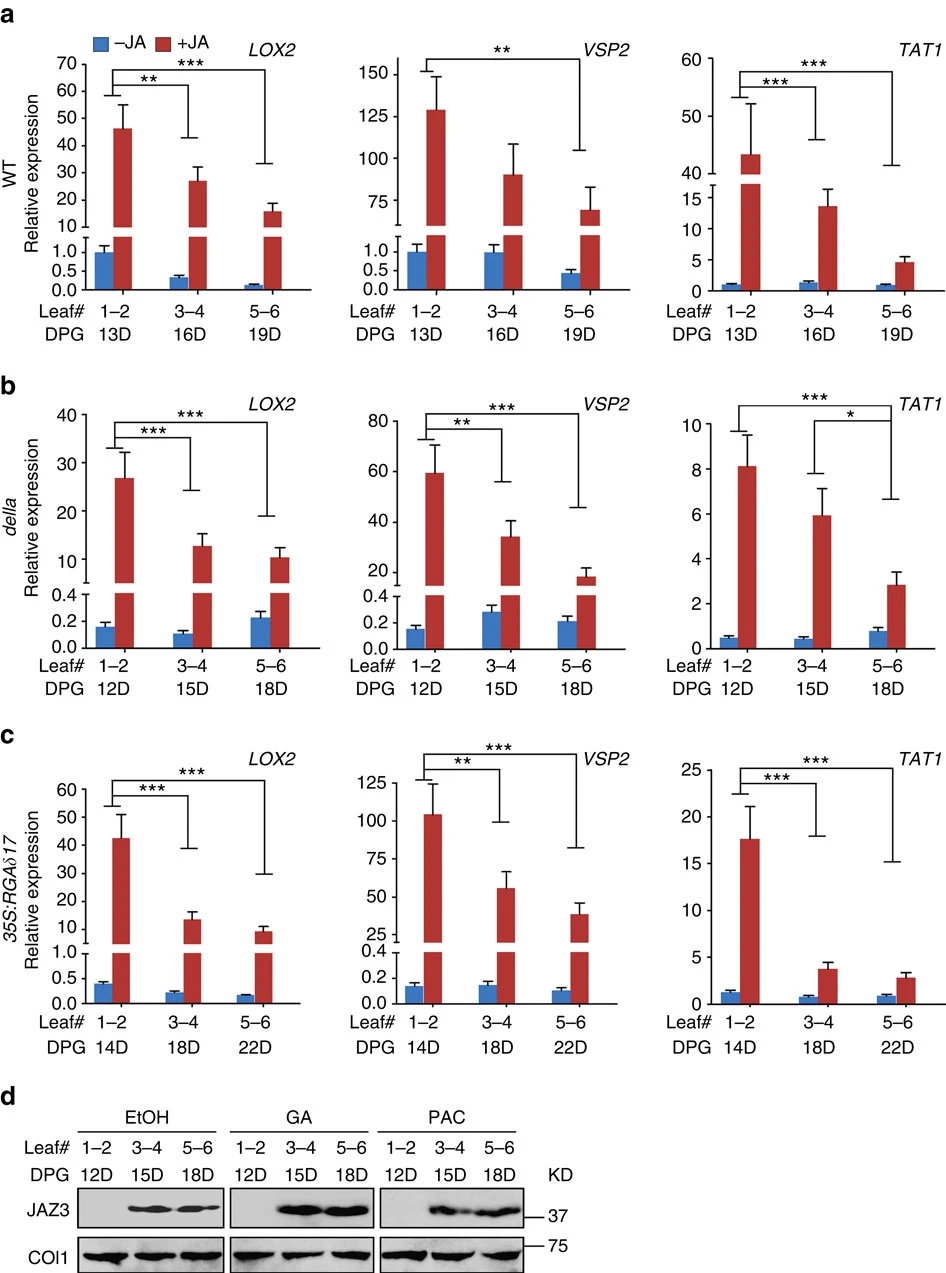
Figure 6. qPCR analyses of the transcript levels of LOX2, VSP2 and TAT1 in leaves of the wild-type (WT) (a), the penta della-deficient mutant (della) (b) and the DELLA over-expressor (35S:RGAδ17) (c) plants. All plants are in Ler-0 background. Plants in LD condition were treated with 50 μM MeJA (+JA) or ethanol (−JA) as control, new leaves (leaf #1–2, 3–4 and 5–6) were harvested at the indicated DPG and analysed. The expression in the first pair of leaves (leaf #1–2) of the wild-type control (−JA) plants was set to 1. Data were analysed by multiple comparisons (Tukey test) followed by two-way ANOVA (*P<0.05, **P≤0.01, ***P≤0.001). Error bars represent ±s.d. (n=3). (d) JAZ3 protein accumulation in GA and PAC treated plants. Ten days after germination, the LD grown 35S:JAZ3-HA plants were sprayed with 50 μM GA or PAC one time per day, and the new leaves were harvested 2 h after the treatment at indicated days (D) post germination. JAZ3-HA was detected using anti-HA antibody. COI1 in each sample was detected using anti-COI1 antibody. ANOVA, analysis of variance; KD, kilodalton.
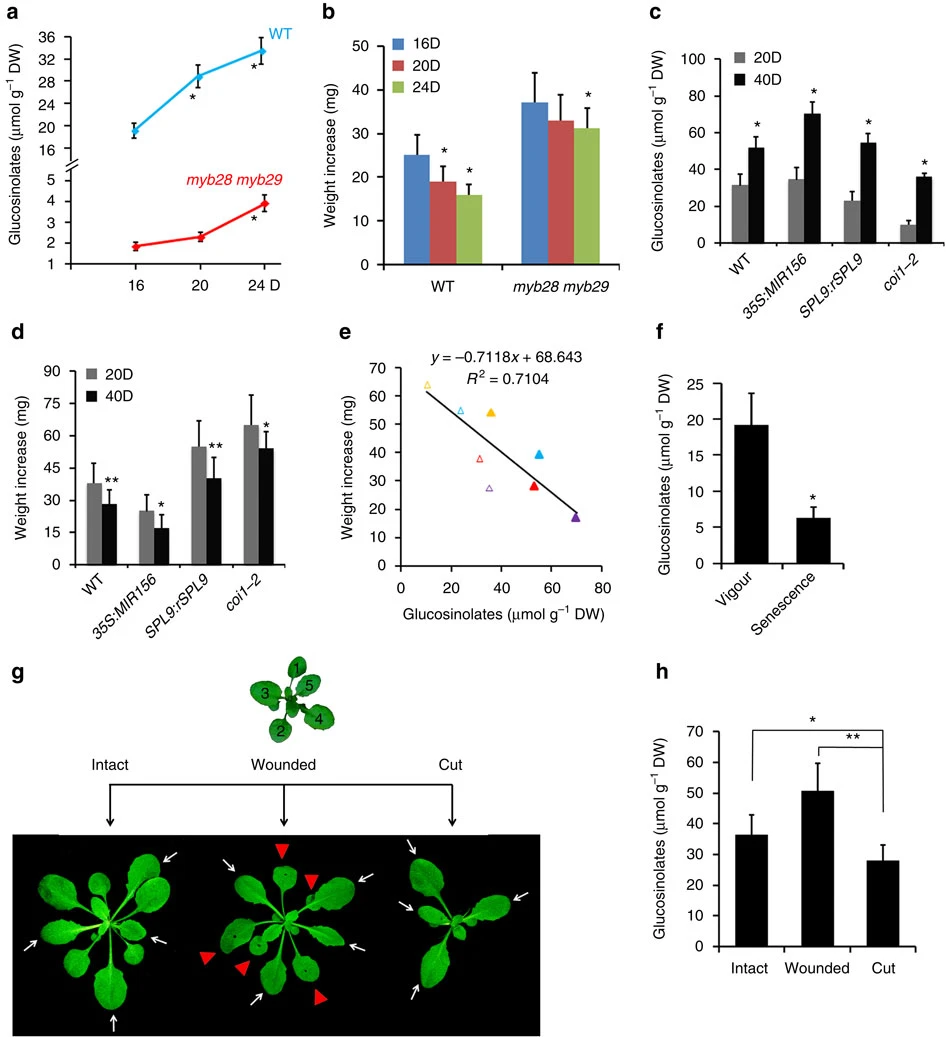
Figure 7. GLSs content was detected by LC-MS (a,c,f,h). Error bars represent ±s.d. (n=3). (a) GLSs content in the wild-type (Col-0) and myb28 myb29. Rapidly expanding leaves of 16-, 20- and 24-day-old plants in LD were used. Asterisk indicates significant difference from the 16-day-old plant (Student’s t-test, *P<0.05). (b) Weight increase of H. armigera larvae fed with leaves described in a. Error bars represent ±s.d. (n=25), asterisk indicates significant difference from the 16D group (Student’s t-test, *P<0.05). (c) GLSs content in rapidly expanding leaves of 20- (20D) or 40-day-old (40D) plants of different genotypes in SD. Asterisk indicates significant difference from the 20D group (Student’s t-test, *P<0.05). (d) Weight increase of H. armigera larvae fed with leaves described in c. Error bars represent ±s.d. (n=25), asterisk indicates significant difference from the 20D group (Student’s t-test, *P<0.05, **P<0.01). (e) Negative relation between GLSs content and larval growth. X axis represents GLSs content in the plant leaves as in c and y axis indicates larval weight increase in d. Weight increase of H. armigera larvae fed with leaves of 20-day-old plant in SD of the wild type (WT), 35S::MIR156, SPL9::rSPL9 and coi1-2 shown as red, purple, blue and yellow hollow triangles, respectively, and those with 40-day-old plant leaves as the respective solid triangles. (f) GLSs decline in leaves during senescence. The first two leaves at vigorous stage (Vigour) from the 14-day-old plants or at senescent (Senescence) stage from the 28-day-old plants in LD were used. Asterisk indicates significant difference from vigour stage (Student’s t-test, *P<0.05). (g,h) Possible mobilization of GLSs from the early leaves to new leaves. The first to fifth leaves from the 20-day-old plant in LD were removed or wounded, four days later the rest four leaves from the plant (cut), the same set of leaves from wounded plants (wounded) and the intact plant (intact) (g) were harvested for GLSs detection (h). White arrows indicate leaves collected for analysis and red triangles refer to wounded leaves. Asterisk indicates significant difference (Student’s t-test, *P<0.05, **P<0.01).
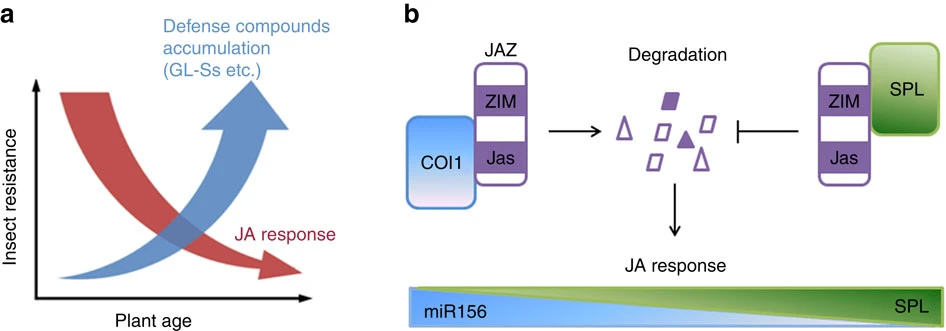
Figure 8. (a) During plant growth, the JA-regulated defense is highly active at juvenile stage but declines with age, while defense compounds accumulate constitutively, which supply the insect resistance continuously during plant growth. (b) The miR156-regulated SPL9 group proteins stabilize JAZs though direct binding to its N-terminal. The increased amount of SPL proteins in aged plant promotes JAZ accumulation, which in turn attenuates JA response.